X‐Ray Crystal Structure of a Second Generation Peptide Dendrimer in Complex with Pseudomonas aeruginosa Lectin LecB
The paper X‐Ray Crystal Structure of a Second Generation Peptide Dendrimer in Complex with Pseudomonas aeruginosa Lectin LecB has been published by Helvetica Chimica Acta.
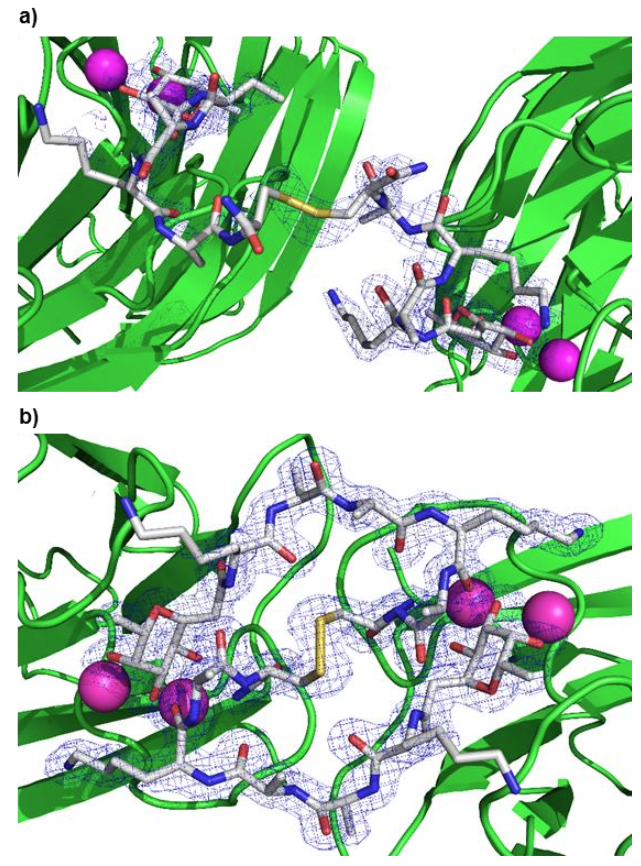
Dendrimers are regularly branched molecular trees which are notoriously difficult to crystallize. Herein we report the crystal structure of a C‐fucosylated second generation peptide dendrimer as complex with lectin LecB in which the only dendrimer‐lectin contact is the LecB bound glycoside (PDB 6S5S). In contrast to a previously reported crystal structure of a first generation peptide dendrimer as LecB complex in which the dendrimer formed trimers connected by intermolecular β‐sheets (PDB 5D2A), the present structure features a globular monomeric state held together by intramolecular backbone hydrogen bonds and assembled into a non‐covalent dimer stabilized by hydrophobic contacts between leucine side‐chains and proline‐phenylalanine CH‐π stacking interactions. Molecular dynamics and circular dichroism studies suggest that this crystal structure resembles the structure of the peptide dendrimer in solution. Structures of an incomplete dendrimer (PDB 6S5R) and of C‐fucosylated disulfide bridged peptide dimers connecting different LecB tetramers are also reported (PDB 6S7G, PDB 6S5P).
Author(s): Stéphane Baeriswyl, Sacha Javor, Achim Stocker, Tamis Darbre, and Jean-Louis Reymond