Spirocyclic Diamine Scaffolds for Medicinal Chemistry
Check out our latest publication Spirocyclic Diamine Scaffolds for Medicinal Chemistry is now published in HELVETICA!
Abstract
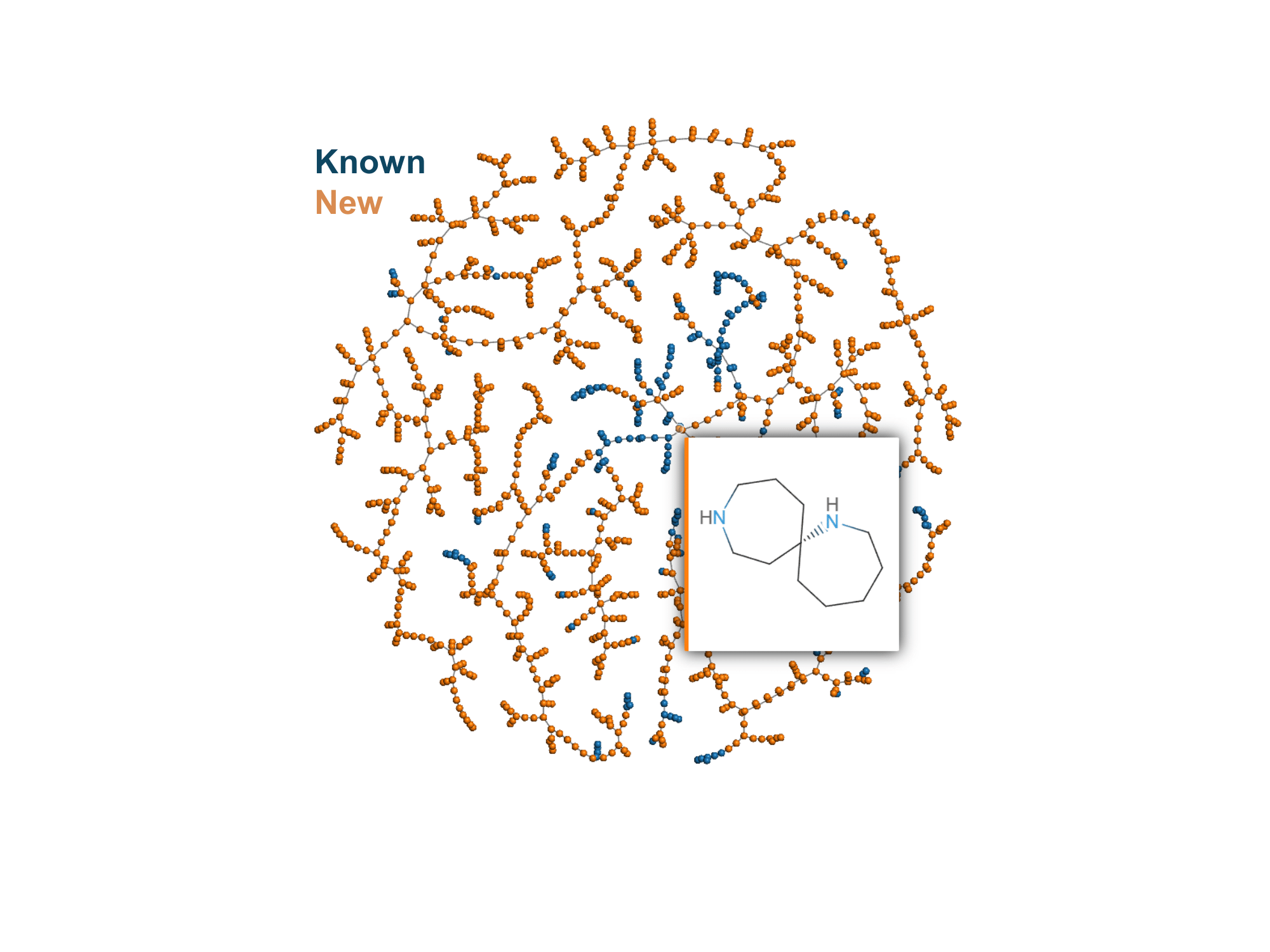
Medicinal chemistry requires the exploration of structurally diverse compound families to expand the repertoire of bioactive small molecule drugs. Here we investigated the potential of spirocycles with ring sizes four to eight carrying a primary or secondary amine in each ring as drug scaffolds. We found that 285 of these 391 spirocyclic diamines were not listed in PubChem and therefore possibly new. Their structural diversity was evidenced by the very low Tanimoto similarity between scaffolds and the presence of up to three stereocenters per scaffold, raising numbers to 1381 possible stereoisomers. To exemplify their use, we prepared four novel spirocyclic diamines containing azepane or azocane rings and tested their activities as inhibitors of potassium channels, neurotransmitter transporters, and muscarinic acetylcholine receptors (mAChR). We identified a single enantiomeric unsubstituted spirocyclic diamine as a micromolar inhibitor of the M4 mAChR.
Author(s) Aline Carrel, Alejandro Flores, Maedeh Darsaraee and Jean-Louis Reymond