Peptide Dendrimers Transfecting CRISPR/Cas9 Plasmid DNA: Optimization and Mechanism
Check out our latest paper Peptide Dendrimers Transfecting CRISPR/Cas9 Plasmid DNA: Optimization and Mechanism in RSC Chemical Biology!
We optimize a peptide dendrimer transfection reagent to deliver a large CRISPR/Cas9 plasmid DNA into cells, elucidate its delivery mechanism, and demonstrate its gene editing efficiency.
Abstract
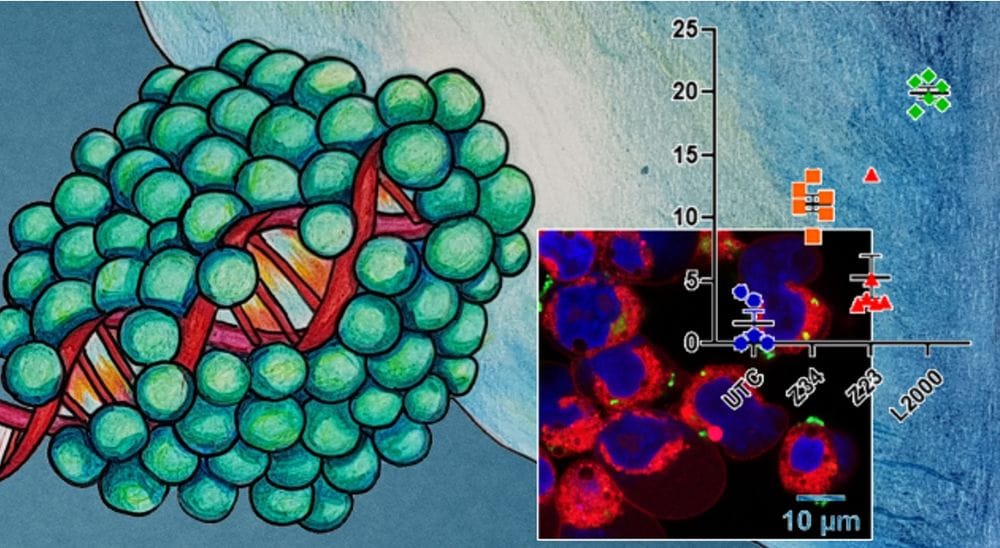
Gene editing by CRISPR/Cas9 offers great therapeutic opportunities but requires delivering large plasmid DNA (pDNA) into cells, a task for which transfection reagents are better suited than viral vectors. Here we performed a structure–activity relationship study of Z22, a D-enantiomeric, arginine containing, lipidated peptide dendrimer developed for pDNA transfection of a CRISPR/Cas9 plasmid co-expressing GFP. While all dendrimer analogs tested bound pDNA strongly and internalized their cargo into cells, D-chirality proved essential for transfection by avoiding proteolysis of the dendrimer structure required for endosome escape and possibly crossing of the nuclear envelope. Furthermore, a cysteine residue at the core of Z22 proved non-essential and was removed to yield the more active analog Z34. This dendrimer shows >83% GFP transfection efficiency in HEK cells with no detrimental effect on cell viability and promotes functional CRISPR/Cas9 mediated gene editing. It is accessible by solid-phase peptide synthesis and therefore attractive for further development.
Author(s): Susanna Zamolo, Elena Zakharova, Lise Boursinhac, Florian Hollfelder, Tamis Darbre and Jean-Louis Reymond