Peptide Dendrimers: From Enzyme Models to Antimicrobials and Transfection Reagents
Read our review article on peptide dendrimers that's published in Chimia:
"Peptide Dendrimers: From Enzyme Models to Antimicrobials and Transfection Reagents".
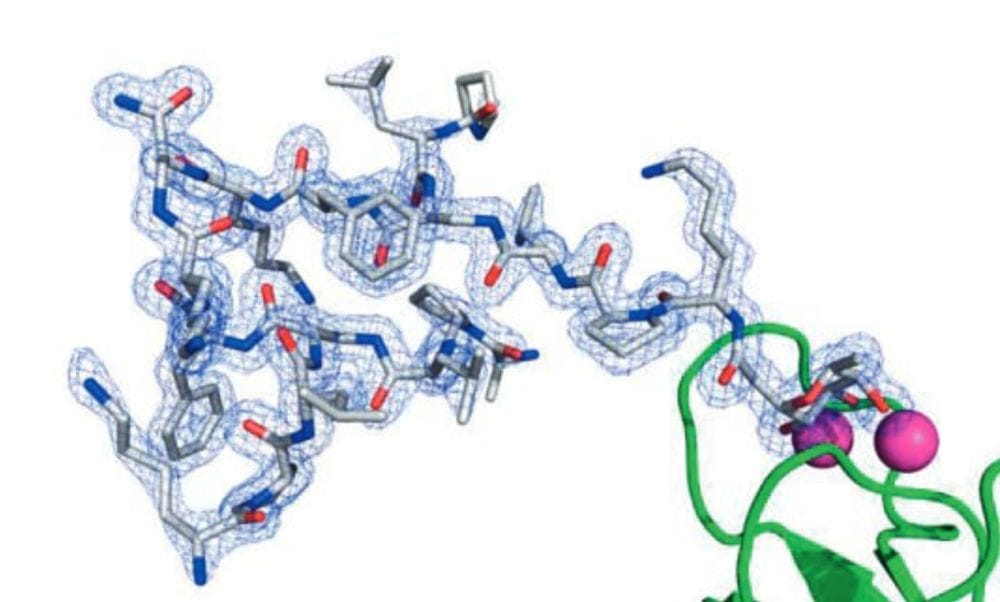
Aiming at studying cooperativity effects between amino acids in easily accessible protein models, we have explored the chemistry of peptide dendrimers, which we obtain as pure products by solid-phase peptide synthesis using a branching diamino acid such as lysine at every second or third position in a peptide sequence, followed by reverse-phase HPLC purification. This article reviews discoveries driven by combinatorial library synthesis and screening, including enantioselective esterase and aldolase enzyme models, cobalamin binding and peroxidase dendrimers, glycopeptide dendrimer biofilm inhibitors and their X-ray crystal structures as complexes with lectins, antimicrobial peptide dendrimers active against multidrug resistant Gram-negative bacteria, and transfection reagents for siRNA and CRISPR-Cas9 plasmid DNA. Latest developments include cheminformatics and artificial intelligence for exploring the peptide chemical space, and the principle of stereorandomization to understand the role of peptide chirality in activity.
Author: Jean-Louis Reymond