Optimizing TRPM4 inhibitors in the MHFP6 chemical space
The paper Optimizing TRPM4 inhibitors in the MHFP6 chemical space has been published by the European Journal of Medicinal Chemistry.
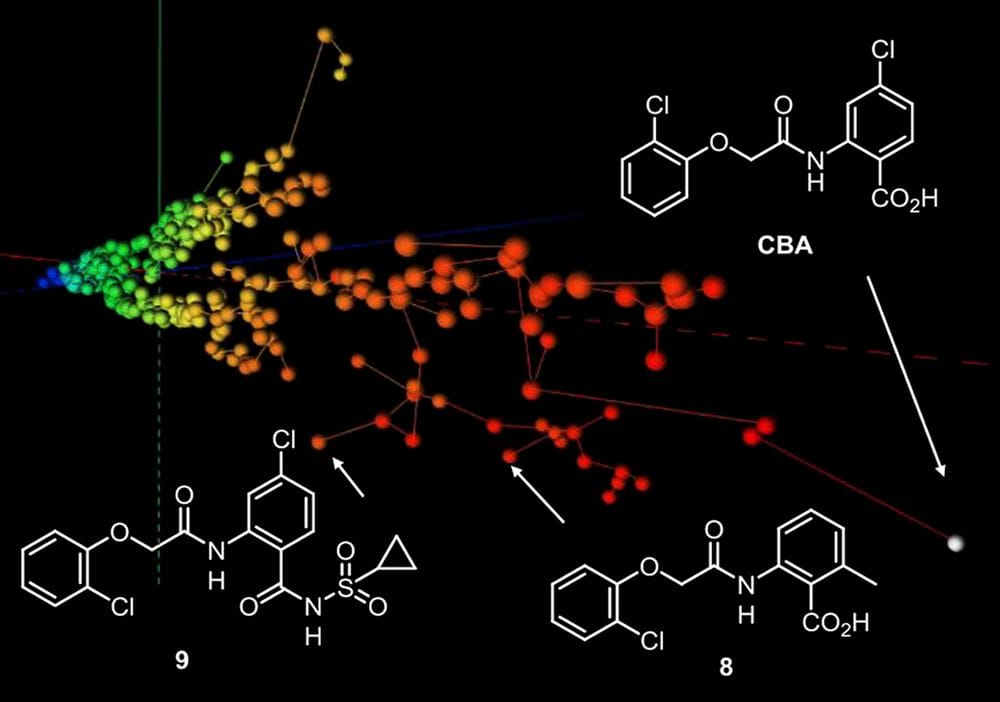
We recently reported 4-chloro-2-(2-chlorophenoxy)acetamido)benzoic acid (CBA) as the first potent inhibitor of TRPM4, a cation channel implicated in cardiac diseases and prostate cancer. Herein we report a structure-activity relationship (SAR) study of CBA resulting in two new potent analogs. To design and interpret our SAR we used interactive color-coded 3D-maps representing similarities between compounds calculated with MHFP6 (MinHash fingerprint up to six bonds), a new molecular fingerprint outperforming other fingerprints in benchmarking virtual screening studies. We further illustrate the general applicability of our method by visualizing the structural diversity of active compounds from benchmarking sets in relation to decoy molecules and to drugs. MHFP6 chemical space 3D-maps might be generally helpful in designing, interpreting and communicating the results of SAR studies. The modified WebMolCS is accessible at http://gdb.unibe.ch and the code is available at https://github.com/reymond-group/webMolCS for off-line use.
Author(s): Clémence Delalande, Mahendra Awale, Matthias Rubin, Daniel Probst, Lijo C. Ozhathil, Jürg Gertsch, Hugues Abriel, Jean-Louis Reymond