Inverse Polyamidoamine (i-PAMAM) Dendrimer Antimicrobials
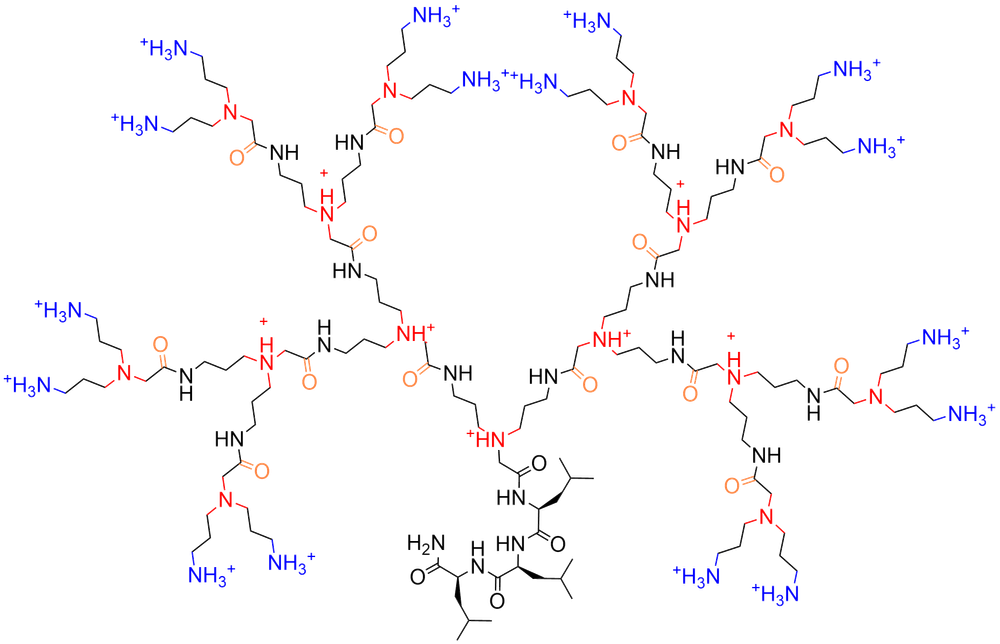
Check out our latest paper Inverse Polyamidoamine (i-PAMAM) Dendrimer Antimicrobials in Helvetica Chimica Acta by Etienne Bonvin! We redesigned polyamidoamine dendrimers by moving the carbonyl across the amide nitrogen to form inverse PAMAMs. Our i-PAMAMs are pure by HPLC, stable, and show potent antibacterial effects.
https://doi.org/10.1002/hlca.202300035
Abstract
Here we redesigned the branches of polyamidoamine (PAMAM) dendrimers by moving the amide carbonyl group on the other side of the amide nitrogen atom, transforming the β-alaninyl-amidoethylamine branch, which easily undergoes retro-Michael reactions and renders PAMAMs intrinsically unstable, into a more stable glycyl-amidopropylamine branch. The resulting inverse PAMAM (i-PAMAM) dendrimers have the same carbon framework as PAMAMs and only differ by the position of the carbonyl group. In contrast to PAMAMs which are prepared in solution and are difficult to purify, we synthesize i-PAMAMs using high-temperature solid-phase peptide synthesis by iterative coupling and deprotection of the commercially available N,N-bis(N'-Fmoc-3-aminopropyl)glycine and purify them preparative reverse phase HPLC. Our i-PAMAM dendrimers show no detectable degradation over time. We demonstrate this new class of dendrimers with the synthesis of antimicrobial dendrimers with potent yet non-membrane disruptive activities against both Gram-negative and Gram-positive bacteria.