Exploring the Sequence Space of Antimicrobial Peptide Dendrimers
Check out our latest paper Exploring the Sequence Space of Antimicrobial Peptide Dendrimers in Israel Journal of Chemistry!
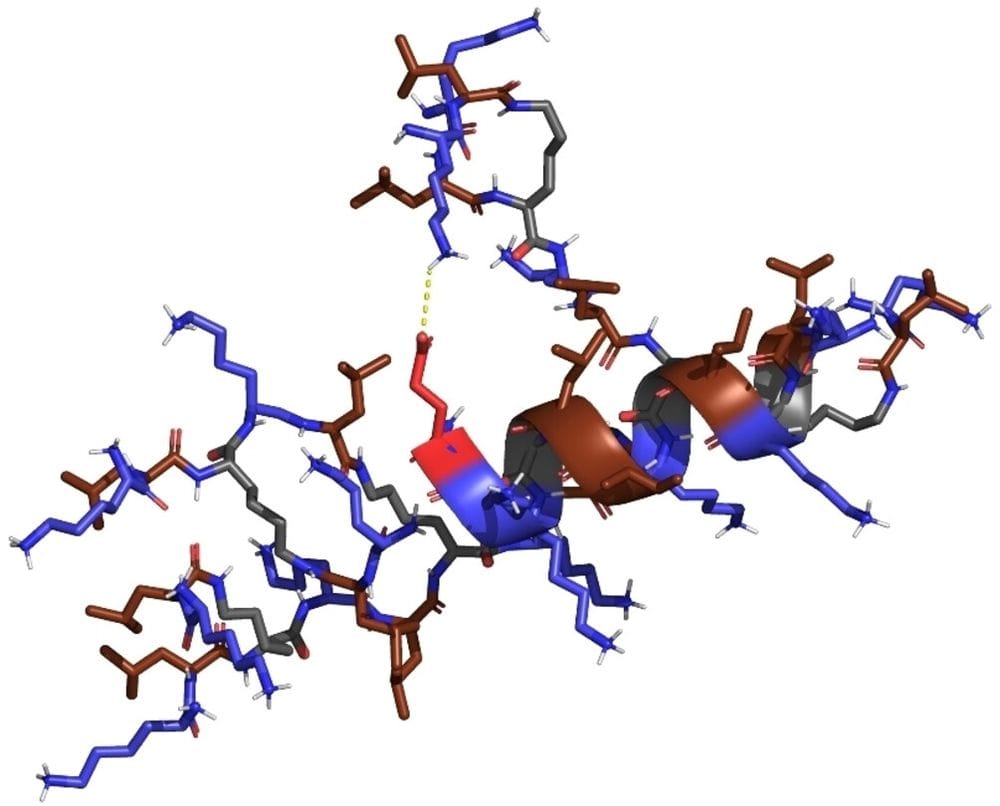
Abstract
There is an urgent need to develop new antibacterial agents against multidrug resistant bacteria. Herein we report our investigation of antimicrobial peptide dendrimers (AMPDs) active against Gram-negative bacteria, whose sequences were designed using a genetic algorithm optimizing molecular similarity to the previously reported AMPD T7 with sequence (KL)8(KKL)4(KKLL)2KKKL. Our computational approach selected analogues unlikely to emerge from a systematic study, including AMPD X66 with a non-conservative Leu→Glu mutation at the dendrimer core which proved compatible with antibacterial effects. Circular dichroism showed that this AMPD is α-helical. Molecular dynamics suggest that its α-helical structure is stabilized by an intramolecular salt bridge involving the core glutamate side chain and a lysine side chain in the dendrimer branches. More substantial variations at the dendrimer core were also tolerated such as the installation of the dianionic pegylated fatty acid side chain of the drug semaglutide potentially useful for in vivo studies.
Author(s): Xingguang Cai, Alice Capecchi, Basak Olcay, Markus Orsi, Sacha Javor, and Jean-Louis Reymond