An intrinsically disordered antimicrobial peptide dendrimer from stereorandomized virtual screening
Our paper An intrinsically disordered antimicrobial peptide dendrimer from stereorandomized virtual screening is published in
Cell Reports Physical Science!
Highlights:
• Stereorandomized antimicrobial peptide dendrimers are identified by virtual screening
• A switch to L residues induces α-helical folding and strong toxicity in one sr-AMPD
• Another AMPD remains intrinsically disordered, active, and non-toxic as L-AMPD
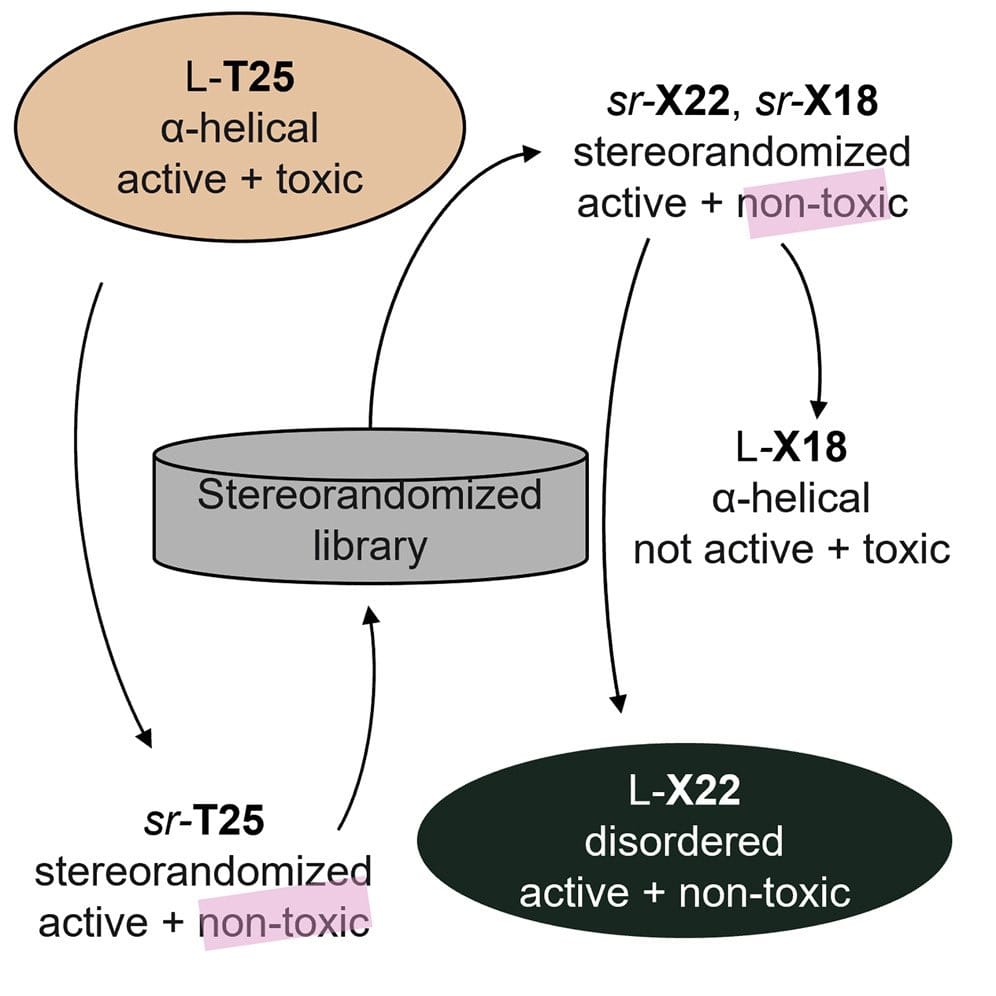
Summary:
Membrane-disruptive amphiphilic antimicrobial peptides behave as intrinsically disordered proteins by being unordered in water and becoming α-helical in contact with biological membranes. We recently discovered that synthesizing the α-helical antimicrobial peptide dendrimer L-T25 ((KL)8(KKL)4(KLL)2KKLL) using racemic amino acids to form stereorandomized sr-T25, an analytically pure mixture of all possible diastereoisomers of L-T25, preserved antibacterial activity but abolished hemolysis and cytotoxicity, pointing to an intrinsically disordered antibacterial conformation and an α-helical cytotoxic conformation. In this study, to identify non-toxic intrinsically disordered homochiral antimicrobial peptide dendrimers (AMPDs), we surveyed sixty-three sr-analogs of sr-T25 selected by virtual screening. One of the analogs, sr-X18 ((KL)8(KLK)4(KLL)2KLLL), lost antibacterial activity as L-enantiomer and became hemolytic due to α-helical folding. By contrast, the L- and D-enantiomers of sr-X22 ((KL)8(KL)4(KKLL)2KLKK) were equally antibacterial, non-hemolytic, and non-toxic, implying an intrinsically disordered bioactive conformation. Screening stereorandomized libraries may be generally useful to identify or optimize intrinsically disordered bioactive peptides.
Author(s):
Xingguang Cai, Markus Orsi, Alice Capecchi, Thilo Köhler, Christian van Delden, Sacha Javor, and Jean-Louis Reymond